XPS and XAES analysis of copper, arsenic and sulfur chemical state in enargite
Enargite, a copper arsenic sulphide with formula Cu3AsS4 is of environmental concern, due to the potential release of toxic arsenic species.
Based on results obtained on natural and synthetic enargite samples and on standards of sulphides and oxides, the Auger parameter α‘ of different compounds was calculated and the Wagner chemical state plots were drawn for arsenic, copper and sulphur. Only sulphur changed from a chemical state similar to that of copper or iron sulfide in freshly cleaved samples to another state in natural enargite in the as received state. Thus, it is the sulphur atom at the surface of enargite that is most susceptible to changes of the enargite surface state and composition. The concept of Auger parameter and chemical state plot used here for the first time for investigating enargite has proved to be a way to unambiguously assign the chemical state of the principal elements copper, arsenic and sulphur in this minerals.
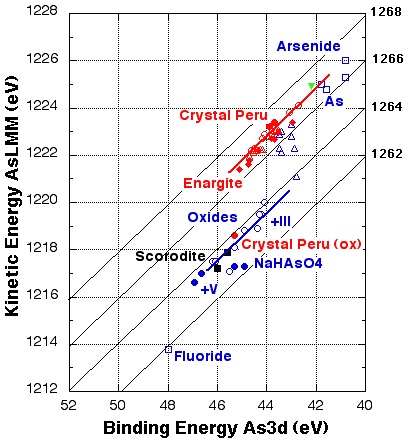
Detailed spectra of the photoelectron and the X-ray induced Auger signals were recorded. A plot of the resulting kinetic energy of the element (e.g. As LMM) versus the binding energy (e.g. As3d) gives the Wagner chemical state plot (see figure). Idential chemical states are found on a diagonal line in the plot.
Based on the chemical state plot arsenic in enargite was found to be in a chemical environment similar to that of arsenides or elemental arsenic whereas copper in enargite is in a chemical state that corresponds to copper sulphide Cu2S for all samples irrespective of surface treatment (natural or freshly cleaved).
The sulfur atom is most susceptible to changes of the enargite surface state and compositioni. A more detailed interpretation of this behaviour, based on differences in the initial and final state effects, is here proposed.
Publications:
M. Fantauzzi, D. Atzei, B. Elsener, P. Latanzi, A. Rossi, XPS and XAES analysis of copper, arsenic and sulfur chemical state in enargite
Surface Interface Analysis 38 (2006) 922 – 930 doi.wiley.com/10.1002/sia.2348