NiP recristallization
From chemical to structural order of electrodeposited Ni24P alloy – an XPS and EDXD study
- In collaboration with Dr Andrzej Królikowski Warsaw Institute of Technology,
- In collaboration with Prof R. Caminiti, Istituto Nazionale di Fisica della Materia, Università “La Sapienza” di Roma
Amorphous electrodeposited nickel-phosphorus alloys with 22 at% of phosphorus (Ni22P) have been analyzed in the amorphous and re-crystallized state by EDXD and XPS surface analysis. The re-crystallization kinetics have been determined following in-situ structural changes by EDXD. Distinct diffraction patterns indicating the presence of Ni3P confirm alloy re-crystallization at 645 °C. The XPS results show that all the core level binding energies of nickel such as Ni2p3/2 and Ni2p1/2 and phosphorus (P2p, P2s) remained constant after the change from x-ray amorphous to crystalline structure of the NiP alloy. Differences observed were: a) the binding energy difference between the Ni2p main lines and the satellite, b) the fine structure of the NiLMM Auger lines and c) the density of states in the valence band in the region of the Ni3d electrons. On the basis of these results from EDXD and XPS it can be concluded that the change in alloy structure from x-ray amorphous to crystalline influences the electronic structure of the NiP alloy but not the chemical state of phosphorous. An explanation based on the screening model proposed in literature is discussed.
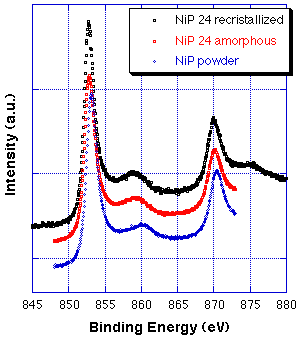
The (re)crystallization kinetics at all three temperatures applied (250, 340 and 645 °C) follows the Liquori’s law, as expected. Only at or above 340 °C a complete re-crystallization was observed, the crystallographic phases formed are similar to crystalline Ni3P.
The XPS Ni2p and P2p core lines as well as the x-ray induced Auger line PKLL do not change in shape and binding energy when the alloy undergoes transition from the amorphous to the fully re-crystallized state. This indicates that re-crystallization does not induce changes in the formal net charge on P and Ni atoms in the alloy. Only the decrease in binding energy difference between the two states in the Ni2p spectra (Ni2p3/2 main peak and its satellite) indicates a different electronic structure of the alloy when changing from x-ray amorphous to crystalline state.
It is hypothesized that the amorphous state shows a higher number of bonding Ni3d electrons respect to the crystallized one. This change in the electronic structure could be verified in the high-resolution valence band spectra of the NiP alloys, where a higher intensity can be noted in the range 4 – 8 eV below the Fermi level. In conclusion the re-crystallization of the electrodeposited amorphous alloy results in a re-arrangement of the nearest neighbors of the P and Ni atoms or in other words in the transition from a chemical order (amorphous) to a structural order.
Publication: B. Elsener, D. Atzei, A. Krolikowski, V. Rossi Albertini, C. Sadun, R. Caminiti, A. Rossi, From chemical to structural order of electrodeposited Ni24P alloy: an XPS and EDXD study, Chem. Mater. 16 (2004) pp 4216 – 4225