Corrosion resistance
The corrosion resistance of electroless deposited nano-crystalline Ni-P alloys
Electroless Ni-P alloys produced as a coating on mild steel in a commercial autocatalytic hypophosphite- type plating bath were studied, with regard to their corrosion behaviour upon immersion in near neutral sulphate and chloride electrolytes. The anodic polarization curves of the alloys showed a current plateau at potentials E < +0.2 V SCE confirming their high corrosion resistance. The dynamic cathodic polarization curves in deaerated solutions showed a pseudo Tafel behaviour with a slope of ca. -0.4 V SCE/decade. Corrosion rates of ca. 0.5 – 0.7 µA/cm2 were found during prolonged immersion in near neutral solutions open to air. Potentiostatic polarization at selected potentials in the range of the current plateau (-0.1 V SCE, +0.1 V SCE) showed a steady decrease of the current density following a power law with exponent -0.5, thus a diffusion controlled process. These results show that the suppression of the anodic dissolution and the low corrosion rates of Ni-P alloys cannot be associated with the oxide-film passivity. Despite this fact, a kind of “localized corrosion” appeared on mechanically polished and “as plated” electroless Ni-P deposits after prolonged potentiostatic polarization at potentials in the range of dissolution suppression.
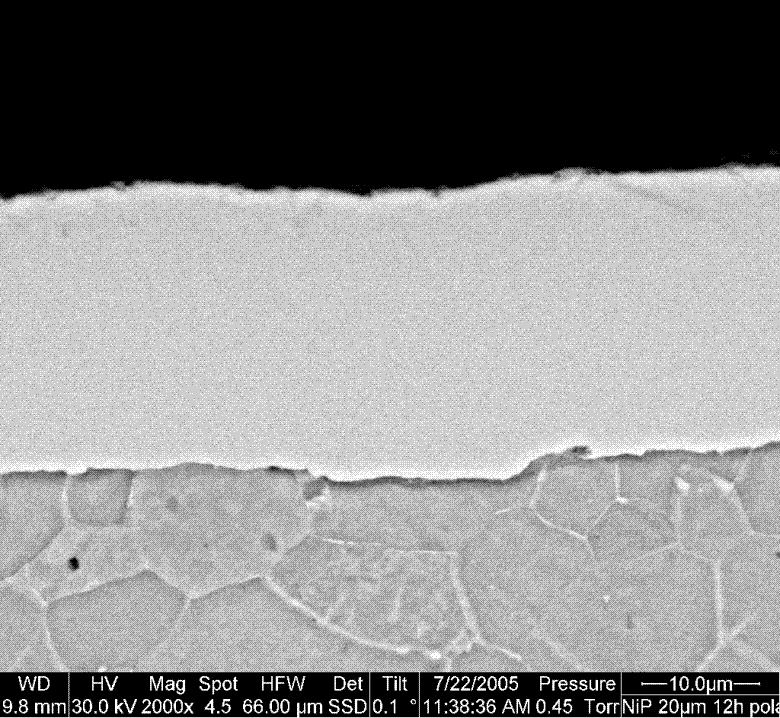
The composition of the NI-P coatings was determined by EDX as 18 – 19 at% phosphorus. Electrochemical experiments (potentiodynamic polarization curves, potentiostatic polarization) were performed in acidic and near neutral solutions both on “as plated” and on mechanically polished samples.
The anodic polarization curves showed a current plateau at potentials E < +0.2 V SCE both in acidic and neutral solutions confirming the high corrosion resistance of the Ni-P alloys. Potetiostatic polarization in the potential range of the plateau showed a steady decrease of the current density following a power law with exponent -0.5, thus a diffusion controlled process.
These results show that the suppression of the anodic dissolution and the low corrosion rates of Ni-P alloys cannot be associated with the normal oxide-film passivity of nickel. The hypothesis of a diffusion control of the nickel dissolution through a phosphorus enriched film at the alloy surface will be tested with XPS surface analysis in a following paper.
Publications:
M. Crobu, Master Thesis at University of Cagliari (2007)
M. Crobu, A. Scorciapino, B. Elsener, A. Rossi, The corrosion resistance of electroless deposited Ni-P alloys, Electrochimica Acta 53 (2008) 3364 – 3370 doi.org/10.1016/j.electacta.2007.11.071