The surface of Enargite after exposure to acidic ferric solutions: an XPS / XAES study
Enargite surface reactivity in acidic ferric solutions simulating acid mine drainage environment has been investigated by X-ray photoelectron (XPS) and X-ray induced Auger (XAES) surface analysis. Natural enargite samples from a Peruvian mine with close on nominal composition Cu3AsS4 have been analysed after cleavage and 24-hour exposure to 0.025 mol/L ferric acidic solutions.
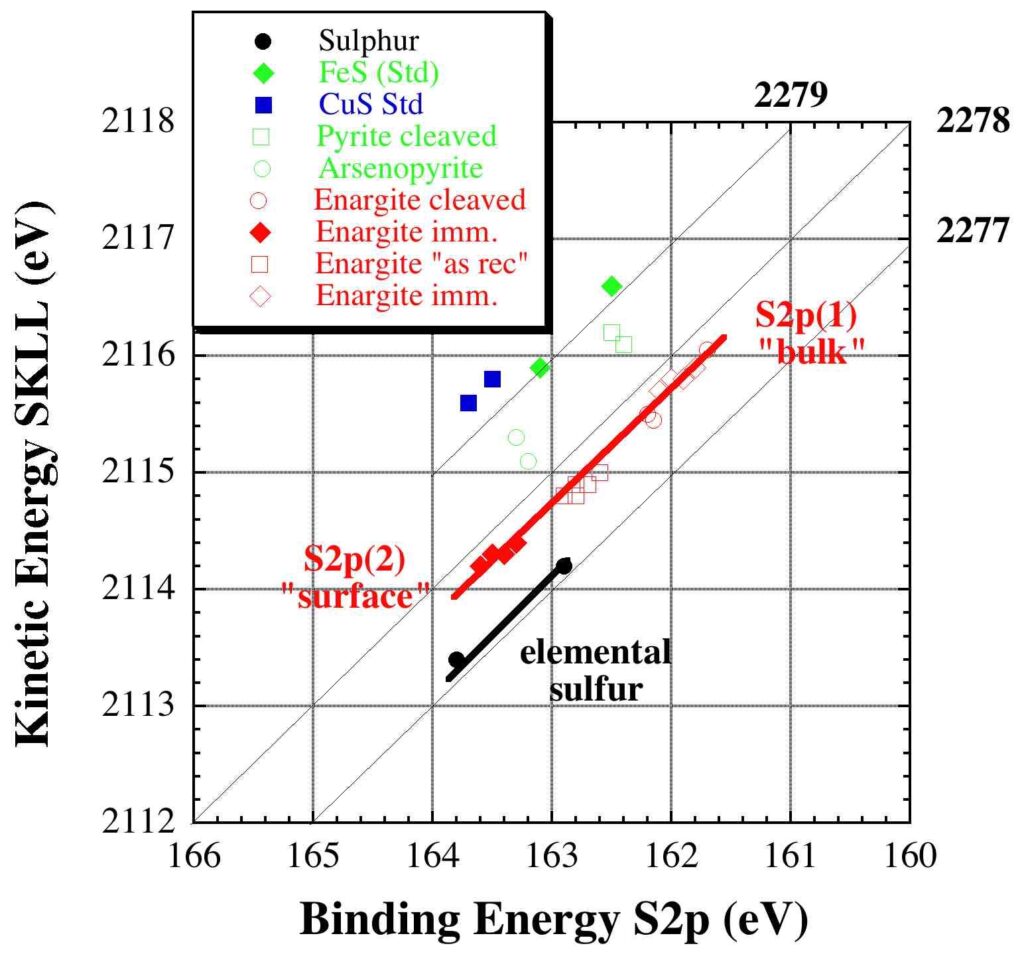
The results, based on the calculated modified Auger parameter α’, show that the chemical state of copper and arsenic in enargite does not change during immersion in acidic, oxidising solutions. The sulphur S2p spectrum of enargite is characterized by two components, one at 162.0 ± 0.2 eV and one at 163.5 ± 0.2 eV. The lower binding energy component (162.0 eV) is attributed to sulphur atoms in enargite. Immersion does not change the chemical state of this compound. XPS/ARXPS measurements show the intensity of the higher binding energy component (163.5 ± 0.2 eV), located at the outermost surface of the reacted enargite, to increase after immersion in solutions. The chemical state plot suggests this sulphur compound might be associated with the formation of a copper-deficient enargite surface layer and not with elemental sulphur.
Based also on the quantitative XPS determinations the surface of the reacted enargite can be described as a layered structure: a thin copper-deficient enargite layer (up to 0.7 nm) is formed on a slightly sulphur enriched enargite surface, the arsenic content remaining constant.
The aim of this work is to investigate the surface of freshly cleaved enargite samples immersed in acidic solutions of FeCl3 (pH = 1.87) and Fe2(SO4)3 (pH=2.04) both containing 0.025mol/L Fe3+ ion, by means of XPS and XAES surface analysis in order to better understand the surface chemistry of enargite during wheathering. This study has implications for the behaviour of enargite in acid mine drainage environments.
XPS surface analysis revealed copper depletion and sulphur enrichment at the enargite surface.
The chemical state of copper and arsenic did not change upon dissolution. The sulphur S2p spectrum showed a sulphur component in the bulk enargite, whose chemical state does not change upon enargite dissolution. Based on the Auger parameter and the chemical state plot, the second sulphur component at higher binding energy, located at the outermost surface, has been assigned to a copper-deficient enargite surface layer formed upon dissolution. The presence of elemental sulphur can be ruled out. Quantitative analysis revealed thickness of this layer to be around 1 nm, thus approximately mono-layer thickness.
Publications:
M. Fantauzzi, B. Elsener, D. Atzei, P. Lattanzi, A. Rossi, The surface of Enargite after exposure to acidic ferric solutions: an XPS / XAES study Surface and Interface Analysis 39 (2007) 908 – 915 doi 10.1002/sia.2607