Study of hydrogen diffusion by electrochemical measurements
Green hydrogen storage and transportation requires steel containers and pipelines that have efficient coatings to prevent hydrogen uptake into steel. This hydrogen permeation barriers (HPB) play a crucial role in preventing the medium to high-strength steel used for storage and transportation infrastructure from hydrogen embrittlement (HE). In the framework of the e.INS WP 7 project “Study of hydrogen permeation barriers for the safe storage and transport of green hydrogen” a procedure (see figure) to determine hydrogen permeation according to Devanathan Starchurski with the electrochemical double cell has been established by the group of Surface Analysis, Electrochemistry and Corrosion.
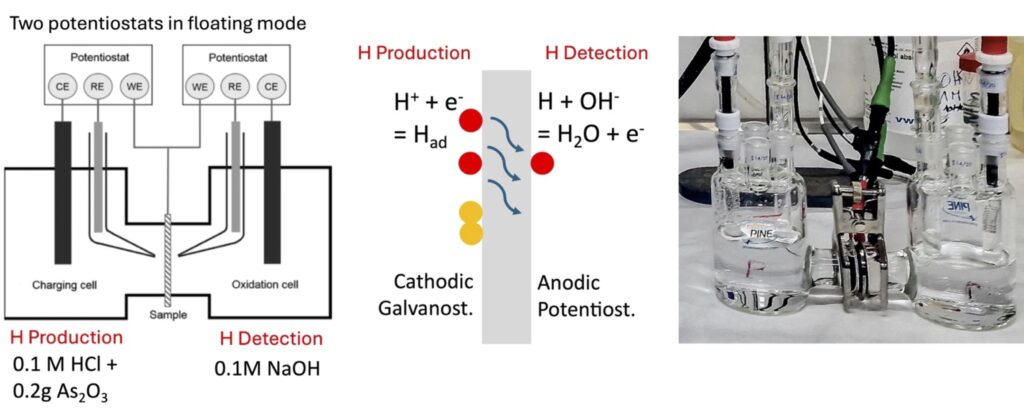
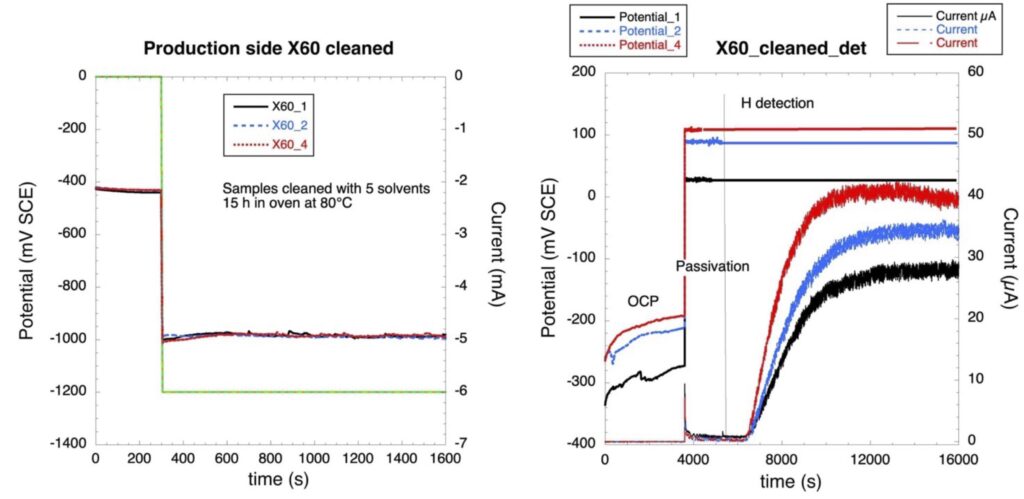
The procedure follows a defined timing both for the hydrogen production and hydrogen detection cell. In the detection cell (figure right) the sample is first exposed to 0.1 N NaOH solution for 1 h at the open circuit potential, then passivated at a potential of OCP + 0.3 V. After one hour of passivation the hydrogen production (figure right) is started at a current of -1 mA/cm2. The hydrogen atoms that absorb and diffuse through the sample are detected by an anodic current in the detection cell.