Mechanism of corrosion resistance of NiP alloys
Electrochemical measurements have shown a diffusion controlled behaviour during potentiostatic tests. Thus the high corrosion resistance of the Ni-P alloys cannot be explained by the classical “oxide film” passivity. XPS/XAES surface analysis after the potentiostatic tests revealed that phosphorus is present in three different chemical states.
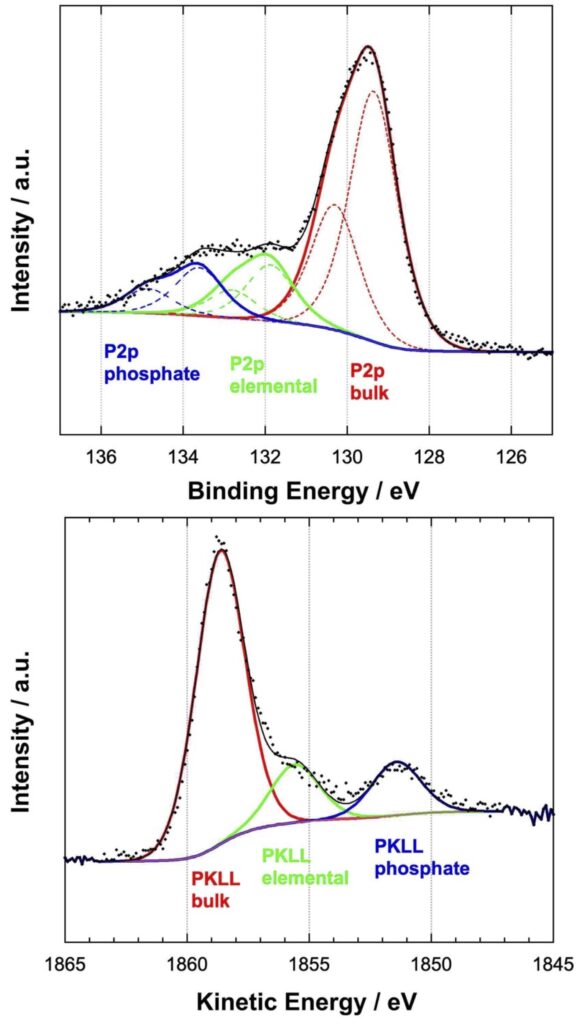
Based on the Auger parameter concept and the chemical state plot, the three chemical states were assigned to:
- a) phosphorus in the bulk alloy (red)
- b) phosphates (blue)
- c) to an intermediate phosphorus compound (green) assigned to elemental phosphorus.
Angle resolved XPS measurements has shown that the elemental phosphorus is enriched at the interface between the bulk alloy and the outermost surface in contact with the corrosive solution. The thickness of this layer is estimated to be about 1 nm, the concentration of phosphorus up to 50%.
The results suggest that the high corrosion resistance of Ni-P alloys can be explained by the strong enrichment of the elemental phosphorus at the interface which limits the dissolution of nickel from the bulk alloy via diffusion mechanism.
Publication: B. Elsener, M. Crobu, A. Scorciapino, A. Rossi, Electroless deposited Ni-P alloys: corrosion resistnace mechanism, J. Applied. Electrochem. 38 (2008) 1053 – 1060, DOI 10.1007/s10800-008-9573-8