
Colorectal cancer
Colorectal cancer (CRC) develops in the epithelium
of the colon or rectum, parts of the large intestine.
Globally, CRC represents the third most common tumor type, accounting for approximately 10% of all cancer cases. In 2018, 1.09 million new cases were diagnosed (more common in men than women). CRC is more common in developed countries, accounting for more than 65% of all CRC cases.
Currently, in patients whose cancer cells are confined within the colon wall, surgery is applied to remove the tumor tissue. In other cases, radiotherapy, chemotherapy or targeted therapy are proposed. In patients with stage III and IV cancer, when tumor cells have spread to lymph nodes or distant organs, chemotherapy with fluorouracil, capecitabine or oxaliplatin is applied.
Available therapies
If the cancer cells have not spread to the lymph nodes, capecitabine (or fluorouracil), irinotecan, oxaliplatin and tegafur/uracil are used.
Antiangiogenic drugs (e.g., bevacizumab) are often used as adjunctive therapy to first-line therapy.
Additionally, epidermal growth factor receptor inhibitors may be used in second-line treatment (e.g., aflibercept, cetuximab, and panitumumab).
All available therapies are intravenous (with the exception of capecitabine which is an oral drug), which requires hospitalization and further increases the overall costs of chemotherapy. Chemotherapeutic strategies, which lack selectivity towards tumor cells, however present various side effects and this sometimes also impacts on the psychological aspect of patients.
A coveted goal for both patients and cancer research is the
development of a small molecule to be administered orally and with high oncological selectivity.
Research steps
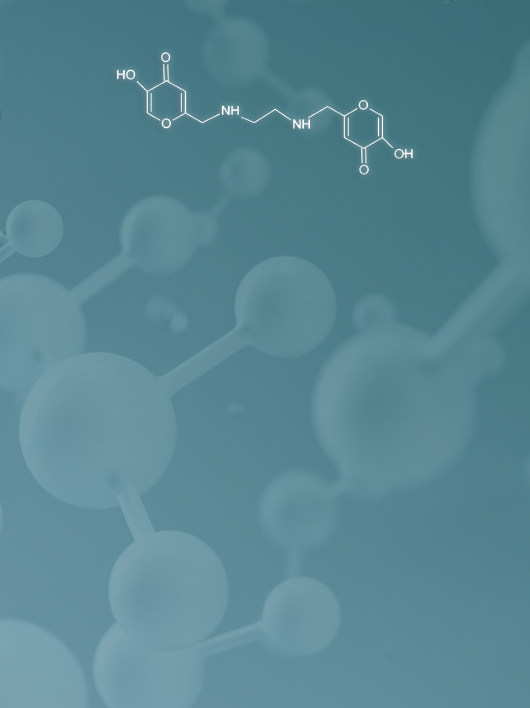
March 24, 2022: a new small molecule (called L1) for oral therapy was patented by our team as a selective inhibitor of mitosis in glioblastoma and CRC cells, which inhibits mitosis and in 24 hours leads to apoptosis of only tumor cells.
During 2023, important developments of the invention have been carried out: the invention was validated in a relevant environment using tumor and non-tumor tissue cultures from the biopsies and/or tumor resections of patients with CRC (AOU Cagliari Ethics Committee, Resolution no. 723 of 09.08.2022).
The study showed that the L1 molecule increases cell death through apoptosis selectively in human tissue tumor cells.
Our invention offers a promising solution to the “selectivity” challenge. The L1 molecule specifically induces apoptosis of tumor cells without the side and unwanted effects related to the non-specificity of the drug. Furthermore, the high solubility in water (>0.6 M) would allow the administration of the molecule orally.
Finally, the synthesis of the L1 molecule respects the practices of green chemistry and is fast (one-step synthesis) and economical (0.45 EUR/10 mg of pure product >99%).
This innovative approach could provide a more effective and convenient treatment option for CRC patients, overcoming the limitations of current therapies.
Project goals and Research Road Map
The main objective of this project is to strengthen the Technological Readiness Level of our invention through further studies on tumor and non-tumor tissues collected from CRC patients. Increasing additional scientific data is necessary to initiate future ethical procedures and funding applications for xenograft model studies and pre-clinical studies.
By increasing the number of scientific data, including immunohistochemical, morphological and molecular data, we expect to identify some molecular mechanisms that are regulated through the pharmacological treatment of CRC with the compound L1. In the context of our project, we will conduct a series of experiments on tumor and non-tumor tissues from patients at the AOU Policlinico of Monserrato. These tissues will be used for immunohistochemical, electron microscopy, molecular and anatomic-pathological analyses at the Departmental Laboratory (Dept. of Medical Sciences and Public Health) and the Institute of Pathological Anatomy.
Our research RoadMap is divided into 4 phases.

Cellular in vitro studies
Completed in 2022

Human biopsies vitro studies
Completed in 2023
Vitro studies with human biopsies with colorectal cancer.
(AOU Cagliari Ethics committee: Resolution no. 723 of 09.08.2022).

Xenograft models
To be completed in 2025

Pre-clinical studies
To be completed in 2026
Applications
Tumor therapy
Our invention could be used for the treatment of tumors, in particular CRC and glioblastoma, both in relapses and metastases, in both humans and animals.
Prevention of tumors
The technology could also be used in the prevention of tumors, both of the intestine and of the glia.
Biochemical study of selective mitotic inhibition
The L1 molecule could be used as a tool for the biochemical study of selective mitotic inhibition in tumor cells.
Impacts

Social
Selective oral therapies that are available at home could reduce social isolation of Colorectal Cancer patients, improving their life quality.

Scientific
Molecular targets and selective pathways open a new line of anti-tumor chemotherapy, and allow to study new preventive actions of CRC.

Economic
Oral therapies reduce patient hospitalization costs. Selective therapies will reduce the cost of insurance and medical treatment of side effects.
Publications
- Pichiri G., Piludu M., Congiu T., Grandi N., Coni P., Piras M., Jaremko M., Lachowicz JI., Kojic Acid Derivative as an Antimitotic Agent That Selectively Kills Tumour Cells, Pharmaceuticals (Basel). 2024 Dec 25;18(1):11. doi: 10.3390/ph18010011. PMID: 39861074; PMCID: PMC11768441.
- J.l. Lachowicz, G. Pichiri, M. Piras, M. Piludu, G. Orrù, P. Coni, T. Congiu, Kojic acid derivative as selective inhibitor of mitosis in colorectal and glioblastoma cancer cells, Italy, 2020.
Patents
- J.l. Lachowicz, G. Pichiri, M. Piras, M. Piludu, G. Orrù, P. Coni, T. Congiu, Kojic acid derivative as selective inhibitor of mitosis in colorectal and glioblastoma cancer cells, Italy, 2020, patent n. WO202258970_A1